Liquefaction of Gases
Liquefaction of Gases: Overview
This topic covers concepts such as Liquefaction of Gases, Andrew's Isotherms, Analysis of Andrew's Isotherms, Andrew Isotherm of Carbon Dioxide, Critical Temperature of Gases, and Expression of Critical Temperature.
Important Questions on Liquefaction of Gases
The cascade method is used to liquify
The Andrew's experiment is the test for the validity of
The critical volume of a gas is . What will be the radius of the molecule in ?
The radius of a molecule is . Calculate the critical volume of the gas.
The critical volume of a gas is . What will be the radius of the molecule in ?
What is critical volume? Give its formula.
Which gas among the following is the easiest to liquify?
The critical temperature of is less than because the molecules have:
The expression of in terms of and is:
The critical volume of a gas when expressed in terms of Van der Waals constants and takes the form:
The van der Waals constant 'a' for different gases are given below:
| Gas | () |
The gas that can be most easily liquefied is
Maximum deviation from ideal gas is expected from:
An ideal gas obeying kinetic theory of gases can be liquefied if
What is the correct increasing order of liquefiability of the gases shown as in the graph below?
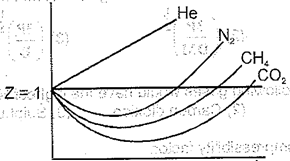
The most favourable conditions to liquefy a gas are:-
The gas with the highest critical temperature is
For a real gas, the curve was experimentally plotted, and it had the following appearance. With respect to liquefaction. Choose the correct statement.
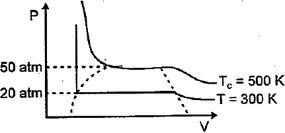
If for the gases, the critical temperature mentioned below i.e.:
| Gas | Critical temp. |
Which of the following can be predicted?
A gas can be liquiefied:
An ideal gas can't be liquefied because :
